Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a) \(n_{Fe}=\dfrac{3,36}{56}=0,06\left(mol\right)\)
PTHH: 3Fe + 2O2 --to--> Fe3O4
0,06->0,04------->0,02
=> mFe3O4 = 0,02.232 = 4,64 (g)
b) VO2 = 0,04.22,4 = 0,896 (l)

\(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\\ pthh:3Fe+2O_2\underrightarrow{t^o}Fe_3O_{\text{4}}\)
0,15 0,1 0,05
\(m_{Fe_2O_4}=0,05.232=11,6\left(g\right)\\
V_{O_2}=0,1.11,4=2,24\left(l\right)\\
pthh:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\uparrow\)
0,2 0,1
\(m_{KMnO_4}=0,2.158=31,6\left(g\right)\)
\(n_{Fe}=\dfrac{8,4}{56}=0,15\left(mol\right)\\ pthh:3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
0,15 0,1 0,05
\(m_{Fe_3O_{\text{ 4}}}=0,05.232=11,6\left(g\right)\\ V_{O_2}=0,1.22,4=2,24\left(l\right)\\ pthh:2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
0,1 0,05
\(m_{KMnO_4}=0,1.158=15,8\left(g\right)\)

\(n_{Fe}=\dfrac{6,8}{56}=0,12mol\)
3Fe + 2O2 \(\underrightarrow{t^o}\) Fe3O4
0,12 0,08 0,04 ( mol )
a, \(V_{O_2}=0,08.22,4=1,792l\)
b, mFe3O4 = 0,04.232 = 9,28g
\(n_{Fe}=\dfrac{6,8}{56}=\dfrac{17}{140}(mol)\\ PTHH:3Fe+2O_2\xrightarrow{t^o}Fe_3O_4\\ a,n_{O_2}=\dfrac{2}{3}n_{Fe}=\dfrac{17}{210}(mol)\\ \Rightarrow V_{O_2}=\dfrac{17}{210}.22,4=1,81(g)\\ b,n_{Fe_3O_4}=\dfrac{1}{3}n_{Fe}=\dfrac{17}{420}(mol)\\ \Rightarrow m_{Fe_3O_4}=\dfrac{17}{420}.232=9,39(g)\)

PTHH: \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
Ta có: \(n_{Fe}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
\(\Rightarrow\left\{{}\begin{matrix}n_{O_2}=0,2mol\\n_{Fe_3O_4}=0,1mol\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{Fe_3O_4}=0,1\cdot232=23,2\left(g\right)\\V_{O_2}=0,2\cdot22,4=4,48\left(l\right)\end{matrix}\right.\)

a) 3Fe + 2O2 Fe3O4
b) nFe = \(\dfrac{8,4}{56}\)= 0,15 mol
nFe3O4 = \(\dfrac{11,6}{232}\) = 0,05 mol
Ta thấy \(\dfrac{nFe}{3}\)= \(\dfrac{nFe_3O_4}{1}\)=> Fe phản ứng hết
<=> nO2 cần dùng = \(\dfrac{2nFe}{3}\)= 0,1 mol
<=> mO2 cần dùng = 0,1.32 = 3,2 gam
c) Oxi chiếm thể tích bằng 1/5 thể tích không khí.
Mà V O2 = 0,1.22,4 = 2,24 lít => V không khí = 2,24 . 5 = 11,2 lít

gọi số mol Fe là x
pthh : 3Fe + 2O2 --t---> Fe3O4
x---->\(\dfrac{2}{3}\)x----------> \(\dfrac{x}{3}\)
=> mFe3O4= \(\dfrac{x}{3}\) . 232 = \(\dfrac{232x}{3}\) (G)
=> VO2 = \(\dfrac{2x}{3}\) . 22,4 = \(\dfrac{224x}{15}\) (L)

a.\(n_{KMnO_4}=\dfrac{m}{M}=\dfrac{31,6}{158}=0,2mol\)
\(PTHH:2KMnO_4\underrightarrow{np}K_2MnO_4+MnO_2+O_2\)
2 1 1 1 ( mol )
0,2 0,1
\(V_{O_2}=n.22,4=0,1.22,4=2,24l\)
b.\(n_{Fe}=\dfrac{m}{M}=\dfrac{11,2}{56}=0,2mol\)
\(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
3 2 1 ( mol )
0,2 0,1
0,1 0,1 0,05 ( mol )
\(m_{Fe_3O_4}=n.M=0,05.232=11,6g\)

nFe = 33,6 : 56 = 0,6 (mol)
pthh : 3Fe + 2O2 -t--> Fe3O4
0,6--> 0,4------->0,2 (mol)
=> vO2 = 0,4.22,4 = 8,96 (mol)
=> mFe3O4 = 0,2.232 = 46,4 (g)
pthh : 2KClO3 -t--> 2KClO3 + 3O2
0,267<-----------------------0,4(mol)
mKClO3= 0,267 .122,5 = 32,67 (g)

a, \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
b, Ta có: \(n_{Fe}=\dfrac{50,4}{56}=0,9\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{2}{3}n_{Fe}=0,6\left(mol\right)\Rightarrow V_{O_2}=0,6.22,4=13,44\left(l\right)\)
c, \(n_{Fe_3O_4}=\dfrac{1}{3}n_{Fe}=0,3\left(mol\right)\Rightarrow m_{Fe_3O_4}=0,3.232=69,6\left(g\right)\)
d, \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
Theo PT: \(n_{KClO_3}=\dfrac{2}{3}n_{O_2}=0,4\left(mol\right)\Rightarrow m_{KClO_3}=0,4.122,5=49\left(g\right)\)
\(n_{Fe}=\dfrac{m}{M}=\dfrac{50,4}{56}=0,9\left(mol\right)\)
\(a.PTHH:3Fe+2O_2\underrightarrow{t^o}Fe_3O_4\)
3 2 1
0,9 0,6 0,3
\(b.V_{O_2}=n.24,79=0,6.24,79=14,874\left(l\right)\)
\(c.m_{Fe_3O_4}=n.M=0,3.\left(56.3+16.4\right)=69,6\left(g\right)\)
\(d.V_{O_2}=14,874\left(l\right)\\ \Rightarrow n_{O_2}=\dfrac{V}{24,79}=\dfrac{14,874}{24,79}=0,6\left(mol\right)\\ PTHH:2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
2 2 3
0,6 0,6 0,9
\(m_{KClO_3}=n.M=0,6.\left(39+35,5+16.3\right)=55,5\left(g\right).\)
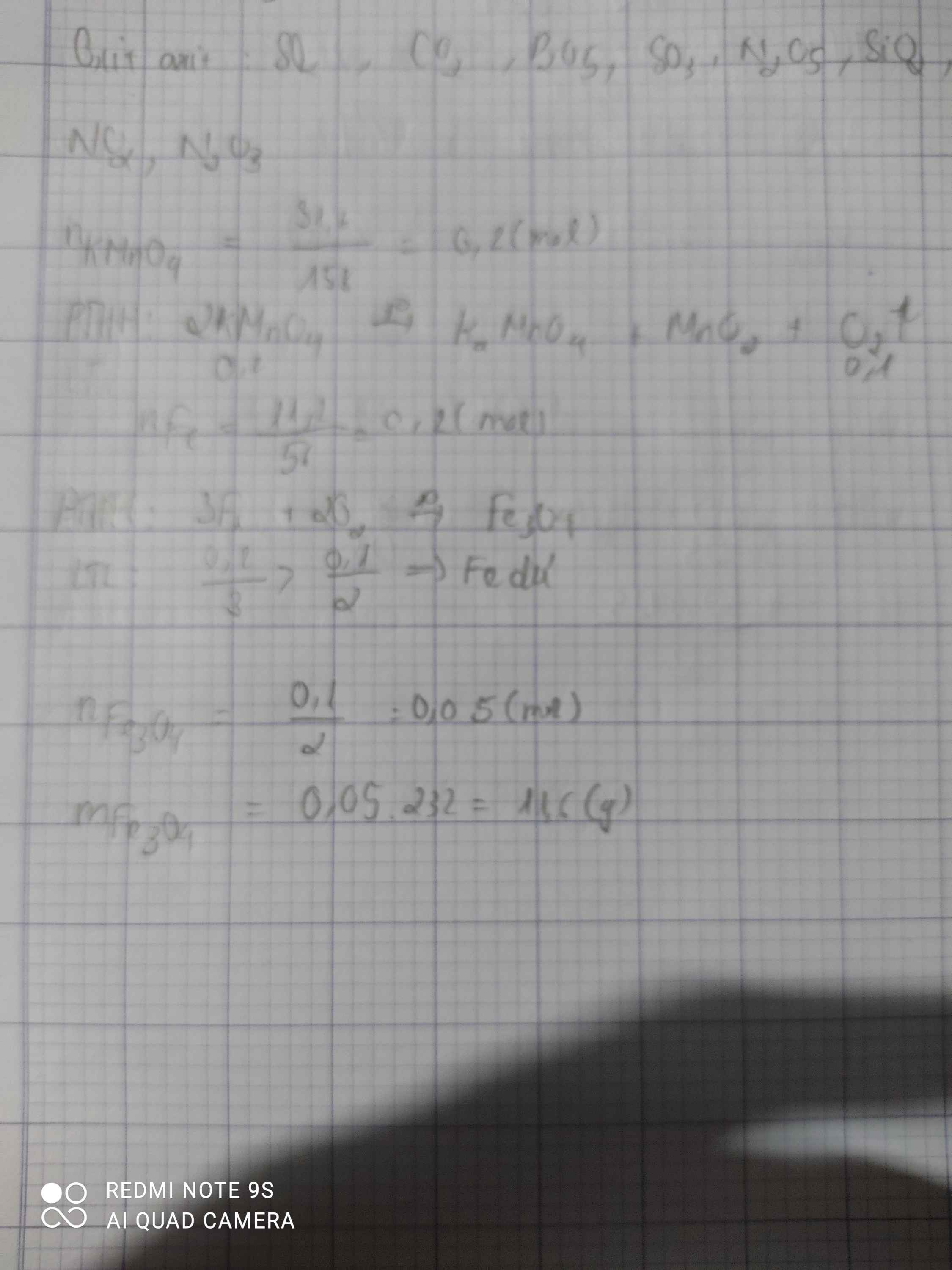
a,
\(n_{KMnO4}=\frac{39,5}{158}=0,25\left(mol\right)\)
\(\Rightarrow n_{O2}=0,125\left(mol\right)\)
\(\Rightarrow V_{O2}=0,125.22,4=2,8\left(l\right)\)
b,
\(n_{O2}=\frac{3,36}{22,4}=0,15\left(mol\right)\)
\(3Fe+2O_2\underrightarrow{^{to}}Fe_3O_4\)
\(n_{Fe3O4}=0,075\left(mol\right)\)
\(\Rightarrow m_{Fe3O4}=232.0,075=17,4\left(g\right)\)
a) 2KMnO4 \(\underrightarrow{t^o}\) K2MnO4 + MnO2 + O2
0,25 -> 0,125 /mol
nKMnO4 = \(\frac{39,5}{158}=0,25\) (mol)
\(V_{O_2}\)= 0,125.22,4 = 2,8 (lít)
b) 3Fe + 2O2 -> Fe3O4
0,15 -> 0,075 /mol
Ta có : 3,36 \(dm^3\) = 3,36l
nO2 = \(\frac{3,36}{22,4}=0,15\) (mol)
mFe3O4 = 0,075.232 = 17,4 (g)