Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

a, \(n_{NaOH}=0,2.1=0,2\left(mol\right)\)
\(m_{NaOH}=0,2.40=8\left(g\right)\)
b, \(n_{H_2SO_4}=2.0,1=0,2\left(mol\right)\)
\(c,C\%=\dfrac{6}{200}.100\%=3\%\)
\(m_{NaCl}=\dfrac{200.8}{100}=16\left(g\right)\)

\(n_{NaOH}=\dfrac{4}{40}=0,1\left(mol\right)\\ C_{M_{NaOH}}=\dfrac{0,1}{0,4}=0,25\left(M\right)\)
n NaOH= \(\dfrac{4}{40}\)=0,1(mol)
C MnaoH=\(\dfrac{0,1}{0,4}\)=0,25(M)

\(1,C_{M\left(HCl\right)}=\dfrac{0,75}{0,5}=1,5M\\ 2,n_{Ca\left(OH\right)_2}=\dfrac{37}{74}=0,5\left(mol\right)\\ C_{M\left(Ca\left(OH\right)_2\right)}=\dfrac{0,5}{1,5}=0,33M\\ 3,n_{NaOH}=0,25+\dfrac{20}{40}=0,75\left(mol\right)\\ C_{M\left(NaOH\right)}=\dfrac{0,75}{2}=0,375M\\ 4,n_{H_2SO_4}=\dfrac{49}{98}=0,5\left(mol\right)\\ C_{M\left(H_2SO_4\right)}=\dfrac{0,5}{2}=0,25M\)

`1) C_[M_[HCl]] = [ 0,75 ] / [ 0,5 ] = 1,5 (M)`
_____________________________________________
`2)n_[Ca(OH)_2] = 37 / 74 = 0,5 (mol)`
`-> C_[M_[Ca(OH)_2]] = [ 0,5 ] / [ 1,5 ] ~~ 0,33 (M)`
_____________________________________________
`3) n_[NaOH] = 0,25 + 20 / 40 = 0,75 (mol)`
`-> C_[M_[NaOH]] = [ 0,75 ] / 2 = 0,375 (M)`
_____________________________________________
`4) n_[H_2 SO_4] = 49 / 98 = 0,5 (mol)`
`-> C_[M_[H_2 SO_4]] = [ 0,5 ] / 2 = 0,25 (M)`

nNaOH=mNaOH/MNaOH = 20/40 = 0,5 mol
200ml = 0,2 lít
CM NaOH = 0,5/0,2 = 2,5 mol/lít
*cái này mình bổ sung

a.\(n_{NaOH}=\dfrac{8}{40}=0,2mol\)
\(V_{dd}=\dfrac{120}{1,2}=100ml=0,1l\)
\(C_{M_{NaOH}}=\dfrac{0,2}{0,1}=2M\)
b.\(n_{NaOH}=\dfrac{21,6}{40}=0,54mol\)
\(V_{dd}=\dfrac{180}{1,2}=150ml=0,15l\)
\(C_{M_{NaOH}}=\dfrac{0,54}{0,15}=3,6M\)

\(a,C_{M\left(NaOH\right)}=\dfrac{0,3}{0,5}=0,6M\\ b,n_{NaOH}=\dfrac{24}{40}=0,6\left(mol\right)\\ C_{M\left(NaOH\right)}=\dfrac{0,6}{0,4}=1,5M\)
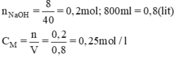
1) ADCT:C%=mct/mdd×100%
→mct=C%×mdd÷100%
→mNaOH=10%×250÷100%=25(g)
2) ta có: nNaOH=20÷40=0,5(mol)
Đổi: 200ml=0,2l
ADCT:CM=n/V
→CMddNaOH=0,5÷0,2=2,5M
3) nNaOH=4÷40=0,1(mol)
ADCT: CM=n/V
→CM=0,1÷2=0,05M
1.
mNaOH=10/100.250=25(g)
2.
nNaOH=20/40=0,5(mol)
=>CM=0,5/0,2=2,5M