Hãy nhập câu hỏi của bạn vào đây, nếu là tài khoản VIP, bạn sẽ được ưu tiên trả lời.

\(n_{C_2H_2}=\dfrac{2,6}{26}=0,1\left(mol\right)\\ 2C_2H_2+5O_2\rightarrow\left(t^o\right)4CO_2+2H_2O\\ a,n_{O_2}=\dfrac{5}{2}.n_{C_2H_2}=\dfrac{5}{2}.0,1=0,25\left(mol\right)\\ \Rightarrow V_{O_2\left(đktc\right)}=0,25.22,4=5,6\left(l\right)\\ b,n_{CO_2}=\dfrac{4}{2}.n_{C_2H_2}=\dfrac{4}{2}.0,1=0,2\left(mol\right)\\ \Rightarrow m_{CO_2}=0.2.44=8,8\left(g\right)\\ n_{H_2O}=n_{C_2H_2}=0,1\left(mol\right)\\ \Rightarrow m_{H_2O}=0,1.18=1,8\left(g\right)\\ \Rightarrow m_{sp}=m_{CO_2}+m_{H_2O}=8,8+1,8=10,6\left(g\right)\)

\(n_{Al}=\dfrac{m}{M}=\dfrac{10,8}{27}=0,4\left(mol\right)\\ a.PTHH:4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
4 3 2
0,4 0,3 0,2
\(m_{Al_2O_3}=n.M=0,2.\left(27.2+16.3\right)=20,4\left(g\right)\\ c.V_{O_2}=n.24,79=0,3.24,79=7,437\left(l\right)\)
\(d.n_{O_2}=\dfrac{m}{M}=\dfrac{12,8}{\left(16.2\right)}=0,4\left(mol\right)\\ PTHH:4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
4 3 2
0,53 0,4 0,27
Tỉ lệ: \(\dfrac{0,53}{4}< \dfrac{0,4}{3}< \dfrac{0,27}{2}\Rightarrow Al_2O_3\) dư và dư \(m_{Al_2O_3}=n.M=0,27.\left(27.2+16.3\right)=27,54\left(g\right).\)
a, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, \(n_{Al}=\dfrac{10,8}{27}=0,4\left(mol\right)\)
Theo PT: \(n_{Al_2O_3}=\dfrac{1}{2}n_{Al}=0,2\left(mol\right)\Rightarrow m_{Al_2O_3}=0,2.102=20,4\left(g\right)\)
c, \(n_{O_2}=\dfrac{3}{4}n_{Al}=0,3\left(mol\right)\Rightarrow V_{O_2}=0,3.22,4=6,72\left(l\right)\)
d, \(n_{O_2}=\dfrac{12,8}{32}=0,4\left(mol\right)\)
Xét tỉ lệ: \(\dfrac{0,4}{4}< \dfrac{0,4}{3}\), ta được O2 dư.
\(\Rightarrow n_{O_2\left(dư\right)}=0,4-0,3=0,1\left(mol\right)\Rightarrow m_{O_2\left(dư\right)}=0,1.32=3,2\left(g\right)\)

a) \(n_{Al_2O_3}=\dfrac{20,4}{102}=0,2\left(mol\right)\)
PTHH: 4Al + 3O2 --to--> 2Al2O3
0,4<--0,3<---------0,2
=> mAl = 0,4.27 = 10,8(g)
b) C1: VO2 = 0,3.22,4 = 6,72(l)
C2: Theo ĐLBTKL: mO2 = 20,4 - 10,8 = 9,6(g)
=> \(n_{O_2}=\dfrac{9,6}{32}=0,3\left(mol\right)=>V_{O_2}=0,3.22,4=6,72\left(l\right)\)
c) Vkk = 6,72 : 20% = 33,6(l)

\(n_{Al}=\dfrac{8.1}{27}=0.3\left(mol\right)\)
\(4Al+3O_2\underrightarrow{^{^{t^0}}}2Al_2O_3\)
\(0.3.....0.225........0.15\)
\(m_{Al_2O_3}=0.15\cdot102=15.3\left(g\right)\)
\(V_{O_2}=0.225\cdot22.4=5.04\left(l\right)\)

Bài 1:
\(a,2Cu+O_2\underrightarrow{t^o}2CuO\)
b, \(n_{O_2}=\dfrac{1,12}{32}=0,035mol\)
\(n_{Cu}=\dfrac{6,4}{64}=0,1mol\)
\(\dfrac{0,1}{2}>\dfrac{0,035}{1}\) => Cu dư, O2 đủ
\(n_{Cu}\left(dư\right)=0,1-0,07=0,039\left(mol\right)\)
c, \(m_{CuO}=0,07.80=5,6g\)
Bài 2:
\(n_{Al}=\dfrac{13,5}{27}=0,5mol\)
\(n_{O_2}=\dfrac{6,67}{32}=0,21\left(mol\right)\)
\(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
\(\dfrac{0,5}{4}>\dfrac{0,21}{3}\) => Al dư, O2 đủ
\(n_{Al_2O_3}=\dfrac{2}{3}.0,21=0,14\left(mol\right)\)
\(m_{Al_2O_3}=0,14.102=14,28g\)

3Fe+2O2-to>Fe3O4
0,225--0,15
n Fe=\(\dfrac{12,6}{56}\)=0,225 mol
VO2=0,15.22,4=3,36l
2KClO3-to>2KCl+3O2
0,1---------------------0,15
=>m KClO3=0,1.122,5=12,25g
\(a,3Fe+2O_2\rightarrow Fe_3O_4\)
\(b,\)
Ta có : \(n_{Fe}=\dfrac{m}{M}=\dfrac{126}{56}=2,25\left(mol\right)\)
\(\Rightarrow n_{O_2}=\dfrac{2}{3}n_{Fe}=\dfrac{2}{3}.2,25=1,5\left(mol\right)\)
\(\Rightarrow VO_2=33,6\left(l\right)\)
\(c,\)
\(PTHH:2KClO_3\rightarrow2KCl+3O_2\)
Theo \(PTHH:n_{KClO_3}=\dfrac{2}{3}n_{O_2}=\dfrac{2}{3}.1,5=1\left(mol\right)\)
\(\Rightarrow m_{KClO_3}=n.M=1,122,5=122,5\left(g\right)\)

a)
nP=6,2/31=0,2(mol)
nO2=6,72/22,4=0,3(mol)
4P+5O2->2P2O5
TPU 0,2 0,3
PU 0,2 0,25 0,1
SPU 0 0,05 0,1
=>Oxi dư
mO2 dư=0,05x32=1,6(g)
b)
P2O5 là chất tạo thành
mP2O5=0,1x142=14,2(g)
\(n_P=\dfrac{6.2}{31}=0.2\left(mol\right)\)
\(n_{O_2}=\dfrac{6.72}{22.4}=0.3\left(mol\right)\)
\(4P+5O_2\underrightarrow{t^0}2P_2O_5\)
\(0.2.....0.25.....0.1\)
\(m_{O_2\left(dư\right)}=\left(0.3-0.25\right)\cdot32=1.6\left(g\right)\)
\(m_{P_2O_5}=0.1\cdot142=14.2\left(g\right)\)

\(n_P=\dfrac{7,44}{31}=0,24mol\)
\(4P+5O_2\underrightarrow{t^o}2P_2O_5\)
0,24 0,3 0,12
\(V_{O_2}=0,3\cdot22,4=6,72l\)
\(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
0,2 0,3
\(m_{KClO_3}=0,2\cdot122,5=24,5g\)
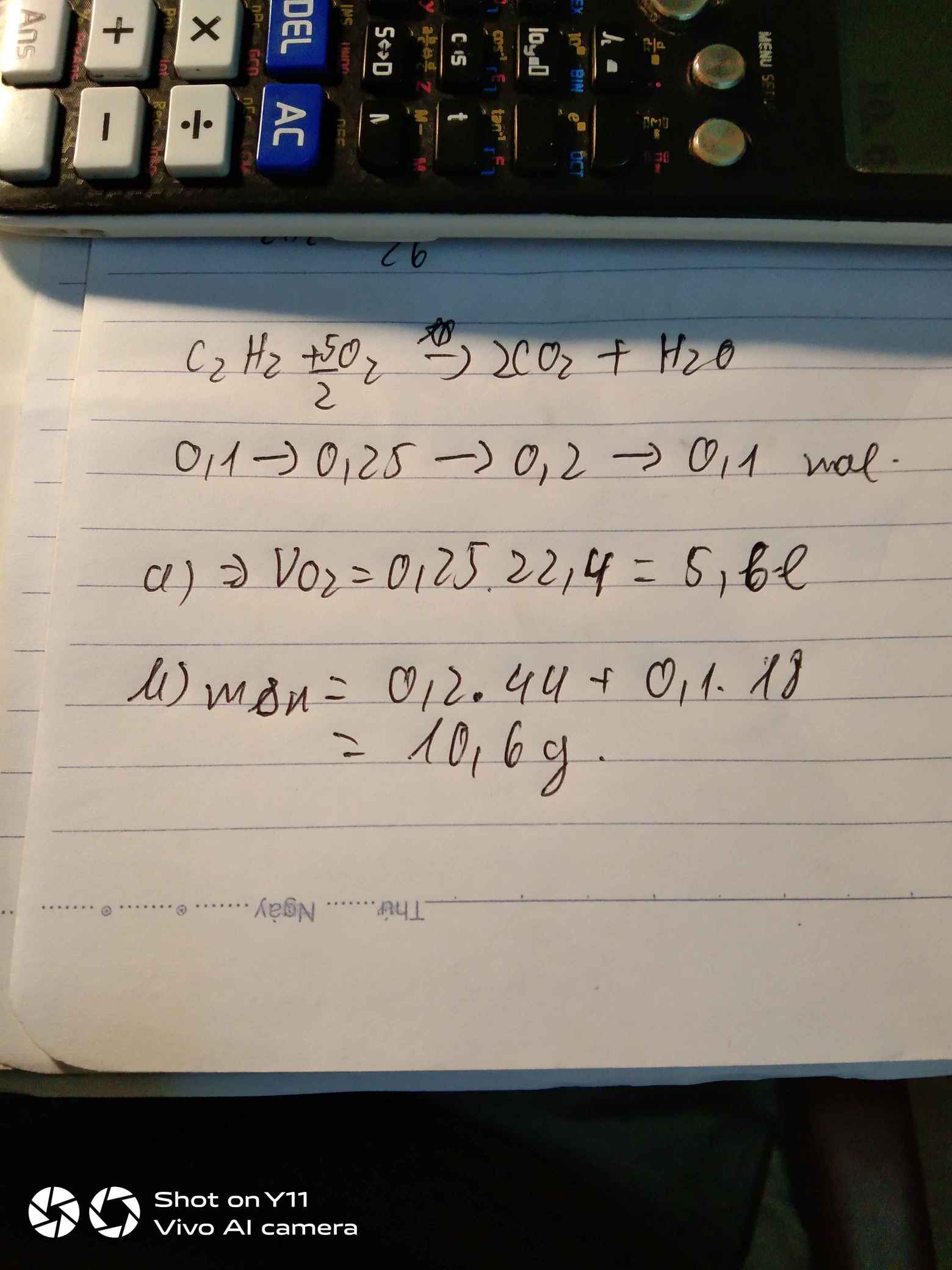
a.\(n_{O_2}=\dfrac{11,2}{22,4}=0,5mol\)
\(n_P=\dfrac{6,2}{31}=0,2mol\)
\(4P+5O_2\rightarrow\left(t^o\right)2P_2O_5\)
0,2 < 0,5 ( mol )
0,2 0,1 ( mol )
\(m_{P_2O_5}=0,1.142=14,2g\)
b.\(n_{O_2\left(dư\right)}=0,5-\left(0,2.5:4\right)=0,25mol\)
\(C+O_2\rightarrow\left(t^o\right)CO_2\)
0,25 0,25 0,25 ( mol )
\(V_{CO_2}=0,25.22,4=5,6l\)
\(m_C=0,25.12=3g\)